With high accuracy of ±0.3℃(Full scope), Freshliance Thermis Log 1D single use USB temperature data logger is an ideal choice for recording and monitoring temperature changes during the clinical drug cold chain.
Ensuring proper environmental monitoring of pharmaceutical storage temperature conditions is crucial for the stability of the products. The conditions should be consistent throughout the supply chain logistics. Poor temperature control can be detrimental to the effectiveness of the drugs. Improper storage conditions at the warehouse or in transit can cost thousands in wasted products.
Monitoring Pharmaceutical Storage Temperature Ranges
Each medication type has recommended storage conditions. Specific storage temperature conditions and ranges are recommended. Adherence to this guarantee their quality and provide the intended efficacy. There are several categories for the temperature ranges of medicines:
·Non-Refrigerated medicines. Stored ambient room temperature between 10°- 25°C. Usually labeled keep under 25°C, though some are stable up to 30°C
·Refrigerated medicines, including vaccines. Stored between 2°-8°C to maintain efficacy. Strict temperature control is required. Any breach of the cold chain could lead to discarding the pharmaceutical product.
·Freezing temperatures between -10°C to -25°C. Necessary for specific drugs and vaccines such as Zoster, Varicella, or MMR.
The Importance of Monitoring Temperature
Improper temperature storage may have different effects on the medications and drugs stored. If temperatures are out of range by a few degrees Celcius their chemical stability can be affected. Worst case it can alter a drug’s physical properties. This can have dangerous consequences when administered to patients. A critical time for pharma products is when in transit to their destination. During transport, they could be in an unpredictable and uncontrolled environment. Temperature-controlled logistics are crucial for product quality and minimizing spoilage.
Our Solution
Freshliance Thermis Log 1D single use USB temperature data logger can be used to record and monitor temperature changes during the clinical drug cold chain. This logger features high accuracy of ±0.3℃ and large capacity of up to 65000 readings. This usb temperature data logger can record the temperature of refrigerated and frozen medicines during their transit gives traceability. Monitoring during cold chain transport and delivery is crucial. In some countries, it is mandatory for data logging to take place during transport. After the trip the completed, you can tear off this USB temperature sensor’s plastic bag, and plug it into the computer. The report with graph and table will be automatically generated. Analyze downloaded data to verify the maintenance of temperature compliance. Temperature data logging gives early detection of degraded lots before reaching patients.
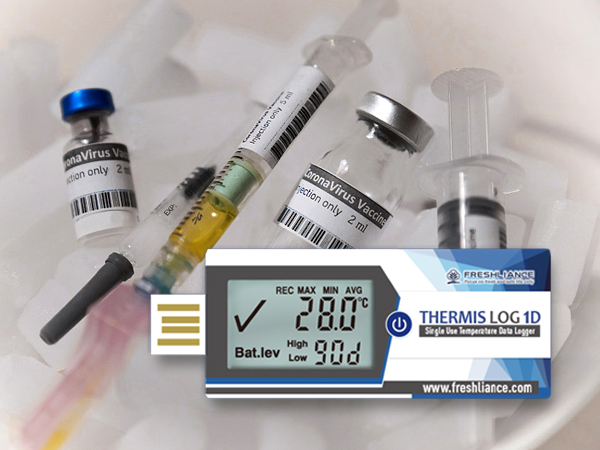
·Model: Thermis Log 1D
·Type: Disposable
·Temperature Range: -30℃~+70℃
·Temperature Accuracy: ±0.3℃ (Full scope)
·Run Days: Max. 90 days
·±0.3℃ High accuracy for pharmaceuticals
·Automatic generation of PDF/CSV reports
·IP67 Professional protection grade
·Pre-programmed parameters for easy operation
·Compatible with Windows and Mac OS alike
·Unique measuring solution based on sensor made in Japan
Contact Us
Freshliance temperature data logger gives precise knowledge of the temperature. Real-time monitoring allows you to be confident in the quality and safety of the drugs. Contact us to get the environmental monitoring solution tailored for your needs.

 English
English Español
Español Русский
Русский Français
Français Deutsch
Deutsch عربي
عربي 中文
中文